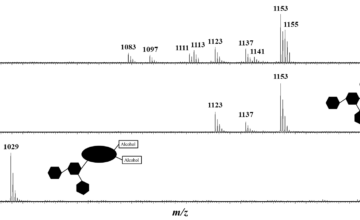
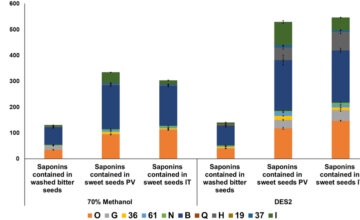
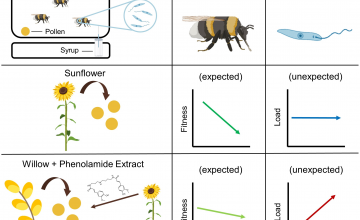
Recherche
New paper in the frame of the ALPO Interreg project
Here, we report the synthesis of a novel diester bearing a pendant lactam unit from methyl levulinate and aspartic acid. The palladiumcatalyzed reductive amination/cyclization sequence was carefully optimized to afford the diacid with high yield (>95 %).
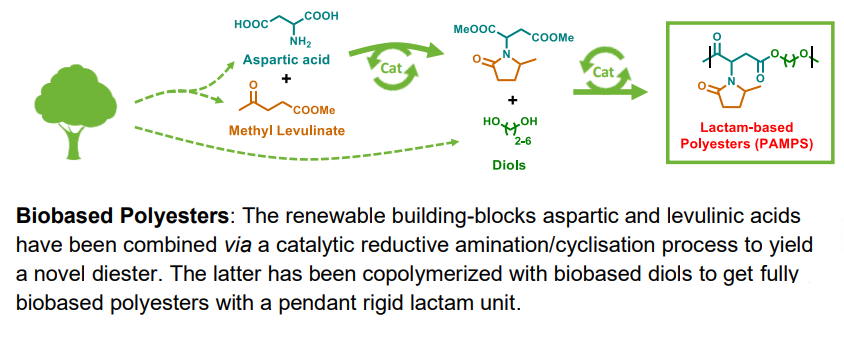
Just accepted in ChemSusChem.
Environmental regulation and fossil resources depletion boost the search for new polymeric materials produced from biomass.
Here, we report the synthesis of a novel diester bearing a pendant lactam unit from methyl levulinate and aspartic acid. The palladiumcatalyzed reductive amination/cyclization sequence was carefully optimized to afford the diacid with high yield (>95 %).
In a second step, the compound was esterified to give the corresponding diester. The latter monomer was copolymerized with α-ω linear diols yielding polyesters with molecular weights up to 20.5 kg.mol-1 .