New paper on Ion Mobility for Natural Product Analysis in collaboration with Waters UK
In collaboration with scientists @ Waters UK, we demonstrated that regioisomeric and stereoisomeric saponins may be distinguished using cyclic ion mobility experiments.
The full paper will be published in the Journal of The American Society for Mass Spectrometry.
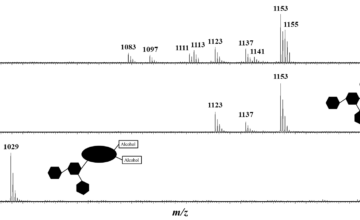
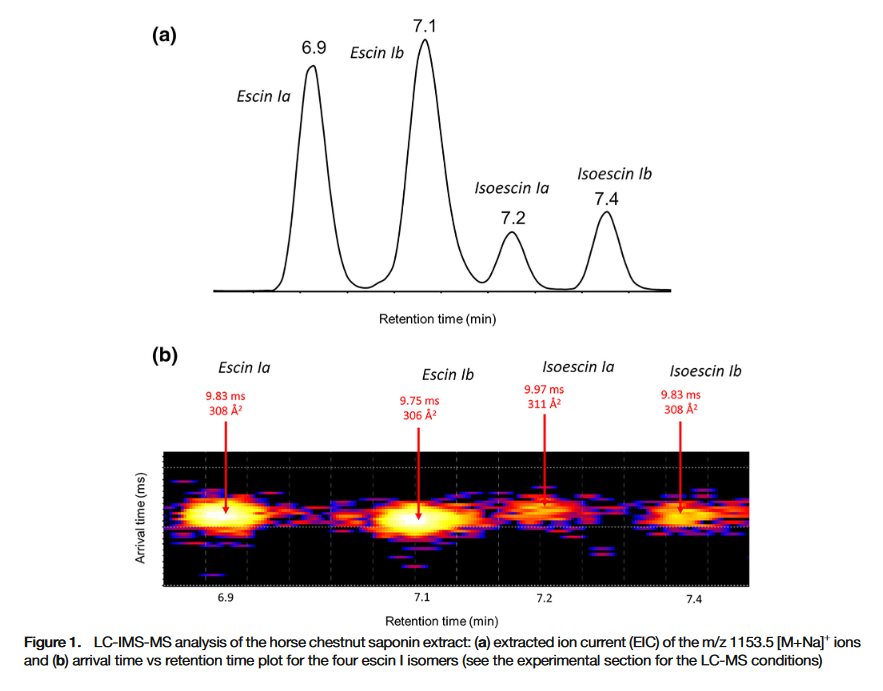
Aesculus hippocastanum, commonly known as the horse chestnut tree or conker tree, is a well-known large tree, abundantly cultivated in streets and parks in temperate countries, especially in western Europe. Its seeds are well-known to contain saponin congeners presenting different compositions and different structures. The HC saponin extract is particularly challenging from an analytical point of view since regioisomeric and stereoisomeric saponins are present. In the present paper, it has been shown that ion mobility experiments together with liquid chromatography separation can be utilized for the structural characterization of stereoisomeric and regioisomeric saponins. Saponins presenting isomeric side chains, such as tiglic and angelic acid residues, can be distinguished by recording the Arrival Time Distributions (ATD), provided that high resolution ion mobility separation is used. This was demonstrated by comparing the capabilities of the Waters SYNAPT G2-Si mass spectrometer equipped with a conventional TWIMS device to an experimental cyclic ion mobility system (cIM) setup. Based on higher ion mobility resolution due to the multi-pass IMSn experiments and versatile fragmentation/spectrum clean-up/ion manipulation capabilities, we succeeded in discriminating stereoisomeric and regioisomeric natural molecules. The present work suggests that natural product analysis will benefit enormously from improvements in ion mobility techniques, in particular increased resolution.
Colson, E., Decroo, C., Cooper-Shepherd, D. et al. J. Am. Soc. Mass Spectrom. (2019).