Just accepted paper: peptoids and hydrogen bonds in gas phase
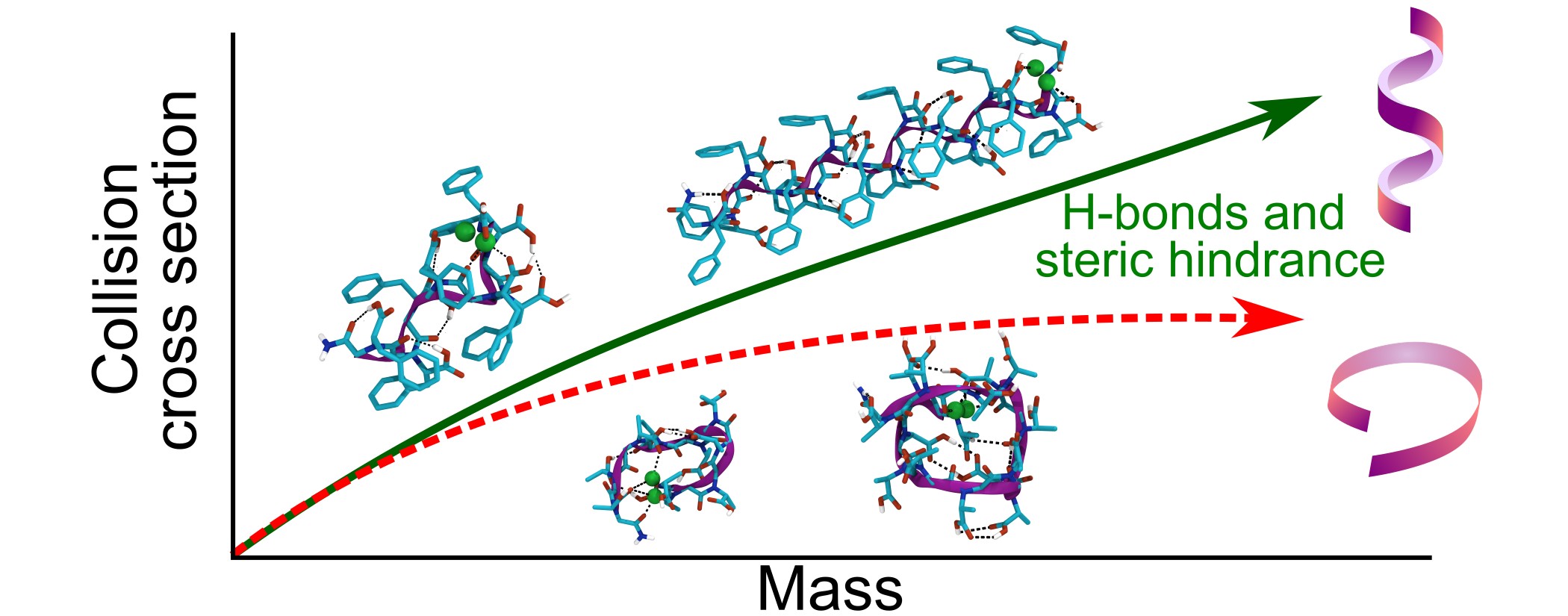
We recently reported a joint experimental and theoretical study of peptoid ions bearing (S)-N-1-phenylethyl side chains. In this study, we demonstrated that the gas phase conformation, probed by ion mobility mass spectrometry, was different from the solution phase conformation (loop vs. helix) because the charge, mandatory for mass spectrometry analysis, must be stabilized and causes the backbone to wrap around to maximize the ion-dipole interactions. In the present study, we introduce another side chain, (S)-N-(1-carboxy-2-phenylethyl), that can form hydrogen bonds. Molecular modeling and ion mobility mass spectrometry of these protonated peptoids strongly suggest that a helical conformation is obtained in gas phase, despite the need to stabilize the charge. This is due to the combination of a hydrogen bond network between the side chains and the backbone carbonyls, and the steric hindrance of the side chains.