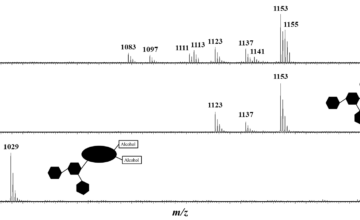
New paper in Collaboration with Sébastien Clément from the University of Montpellier on the synthesis of conjugated semiconducting polymers.
Chevrier et al, Synthetic Metals 252 (2019) 127-134.
A poly(3-hexylthiophene)-block-perfluoropolyether-block-poly(3-hexylthiophene) (P3HT-b-PFPE-b-P3HT) triblock copolymer was synthesized by copper(I)-catalyzed alkyne–azide cycloaddition. The copolymer was unequivocally characterized by size exclusion chromatography, mass spectrometry and nuclear magnetic resonance (NMR). Such a triblock copolymer exhibits i) a good thermal stability with no significant weight loss until 320 °C under air, ii) a glass transition of -50 °C due to the soft PFPE segment and iii) a melting peak arising from P3HT at 236 °C. The UV–vis absorption spectrum exhibits a similar absorption profile to that of pure P3HT with a vibronic structure. Analysis of terpolymer thin films by atomic force microscopy imaging and grazing incidence wide-angle X-ray scattering reveals the formation of P3HT crystallites and an “edge-on” orientation of the polymer chains towards the substrate. Preliminary photovoltaic studies on blends of this triblock copolymer combined with a fullerene derivative indicate that the devices using P3HT-b-PFPE-b-P3HT show an almost similar power conversion efficiency to commercial P3HT.