New paper on the synthesis of poymers for organic radical batteries.
Just accepted in Polymer Chemistry.
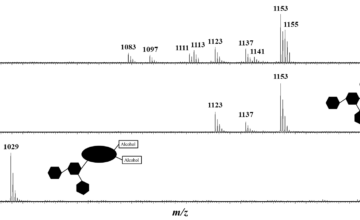
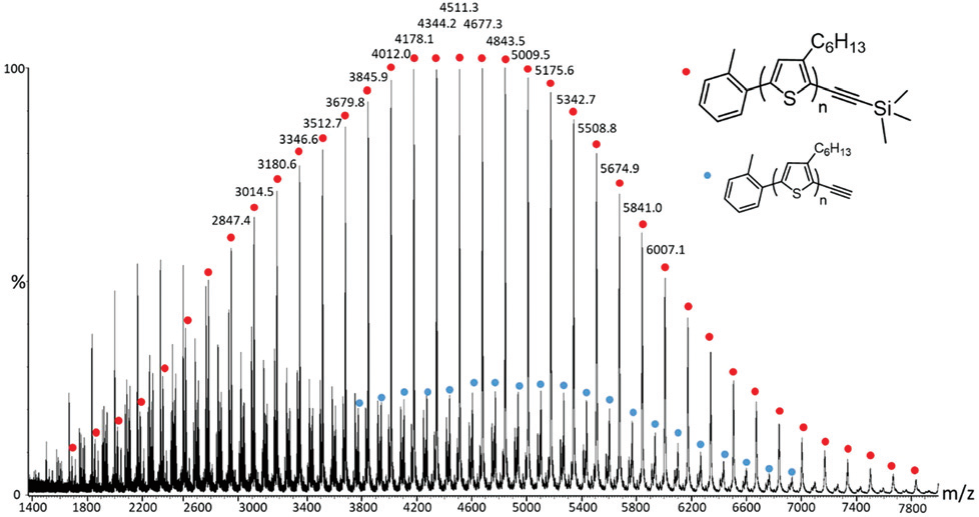
End-functionalized poly(3-hexylthiophene) (P3HT) and poly(2,2,6,6-tetramethylpiperidinyloxy-4-yl methacrylate) (PTMA) were synthesized using controlled polymerization techniques and linked together to lead to a diblock copolymer structure via click cycloaddition. The efficient preparation of the polymers was attested by Nuclear Magnetic Resonance and Mass Spectrometry. These diblock copolymers were further blended with carbon nanotubes (CNTs) to be used as cathodes in organic radical batteries. The performances of these batteries were studied during charge/discharge cycling at constant and variable current densities (C rate). In order to shed light on the importance of the diblock copolymer architecture, the results were compared to the capacities measured on electrodes made of blends of P3HT and PTMA homopolymers. Theoretical simulations were finally used to corroborate the good electrochemical properties of the synthesized diblock copolymers.